CMV Purified Virus Lot I1857119
| Virus Strain | AD169 |
| Host Cell | Normal Human Dermal Fibroblast (NHDF) |
| Cell Line Characterization | Confirmed Negative for HIV and Hepatitis B |
| Purity | Density gradient purified |
| Inactivation | Verified by tissue culture infectivity assay. |
| Supplied In | 10 mM Tris, 100 mM NaCl, 1 mM EDTA, 0.1% Triton X-100 pH 8 |
| Protein Concentration | 1.0 mg/ml |
| Fill Volume | 103 µl (CV046-100) or 1.03 ml (CV046-1) |
| ELISA (IgG) Titer | >1:6400 |
| Western Blot Titer | 10 µg/cm |
| Reference and/or Additional Reading | J. Infectious Diseases (1987), 156(4):615 |
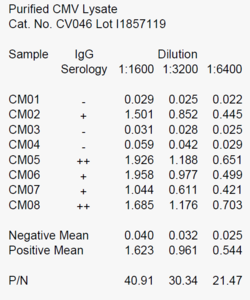
ELISA Results
Antigen is Virusys # CV001 CMV Infected Cell Extract coated at various dilutions in PBS. Primary antibodies (CM01 - CM08) are human plasmas previously characterized for CMV IgG serology.
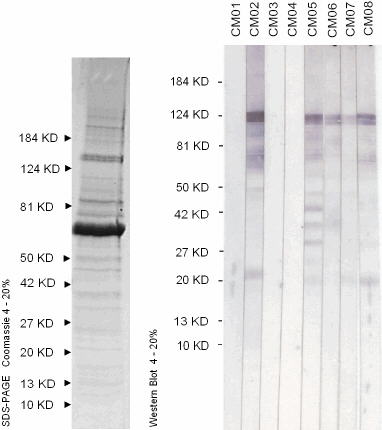
SDS-PAGE and Western Blot Results
Antigen is Virusys # CV046 Gradient Purified CMV at 10 µg/cm. Primary antibodies (CM01 - CM08) are human plasmas previously characterized for CMV IgG serology. See SOP-128 for procedure.
This product is intended for research and manufacturing uses only. It is not a diagnostic device. It is supplied frozen on dry ice - upon receipt, store at -70°C. Product degradation will result from multiple freeze/thaw cycles. It is suggested that the antigen be stored in use size aliquots and thawed just prior to use. Storage at 2 - 8°C for up to twelve hours is acceptable for unused portions.