EBV Information
Epstein-Barr Virus (HHV-4) has a dual cell tropism for human B-lymphocytes (generally non-productive infection) and epithelial cells (productive infection). There is no suitable animal host, but replication/latency has been studied extensively in transformed human cell lines. HHV-4 is widespread worldwide, with ~75% of adults infected (lifelong!).
Virions enveloped; slightly pleomorphic; spherical; 120-220 nm in diameter. Surface projections of envelope small (surface appears rough); spikes; dispersed evenly over all the surface. Nucleocapsids isometric. Nucleocapsid surrounded by the tegument that consists of globular material which is frequently asymmetrically distributed and may be variable in amount. Nucleocapsids sometimes penetrated by stain (although intact envelope impermeable to stain); 100-110 nm in diameter. Symmetry icosahedral. Nucleocapsids appear to be angular. Surface capsomer arrangement obvious. 162 capsomers per nucleocapsid (capsomeres hexagonal in cross-section with a hole running half-way down the long axis). Core consists of a fibrillar spool on which the DNA is wrapped. The ends of the fibers are anchored to the underside of the capsid shell. Incomplete virus particles often present; they are capsids lacking the envelope.
Virions contain one molecule of linear double stranded DNA.
Total genome length is 170000 nt. Genome sequence has terminal repeated sequences; reiterated, but not repeated internally; repeated at both ends. Guanine + cytosine ratio 56 %. Each virion contains full length copy, or shorter copies (some isolates lack as much as 15000 at specific sites).
The usual outcome of infection is polyclonal B-cell activation and benign proliferation, which may be sub-clinical or produce infectious mononucleosis (glandular fever):
|
There is a well-established relationship between HHV-4 and oncogenesis - Burkitts lymphoma and nasopharyngeal carcinoma. Infection is also associated with B cell lymphomas in immunosuppressed patients, certain T cell lymphomas, and Hodgkin's disease.
HHV-4 is a powerful transforming agent for normal resting human B lymphocytes in vitro, resulting in the outgrowth of HHV-4-immortalized lymphoblastoid cell lines (LCL). HHV-4 gene expression in LCL is restricted to about nine of the approximately 100 genes encoded by the virus, and since LCL are largely non-permissive for virus production, the HHV-4 genes expressed in LCL are defined as latent genes. Six of the latent genes encode the nuclear antigens:
- EBNA-1
- EBNA-2
- EBNA-3A
- EBNA-3B
- EBNA-3C
- LP
while three encode the latent membrane proteins:
- LMP-1
- LMP-2A
- LMP-2B
The most abundantly expressed latent transcripts in LCL are non-polyadenylated RNA, the EBERs, which do not encode proteins, but are involved with avoidance of interferons.
EBNA-1, EBNA-2, EBNA-3A, EBNA-3C and LMP-1 are essential for HHV-4-induced transformation of B cells, while EBNA-LP and LMP-2A enhance the transformation efficiency. The process of cellular transformation by HHV-4 is not completely understood. In HHV-4-associated malignancies, not all of the transforming proteins are expressed: in Burkitt's lymphoma, only EBNA-1 is regularly detected, while in nasopharyngeal carcinoma, EBNA-1 is the only protein invariably expressed but many tumours also express LMP-1 and LMP-2. A number of reasons may be put forward to explain these observations:
- A "hit-and-run" hypothesis, in which transforming genes are expressed early in the oncogenic process, but longer required and downregulated in the final tumour
- Genetic aberrations have replaced the requirement for some of the HHV-4 transforming genes, e.g. chromosome translocations in Burkitt's lymphoma which result in deregulated expression of the c-myc gene
- Different sets of HHV-4 genes may be required for transformation of cell types other than B cells, e.g. the epithelial cells in nasopharyngeal carcinoma
Cellular transformation by HHV-4 is a complex process involving the co-operative interactions between several viral proteins.
X-linked lymphoproliferative syndrome (XLP) is a rare condition usually seen in males where on initial infection with HHV-4 (usually around the age of 2-3) results in a hyperimmune response, sometimes causing a fatal form of glandular fever and sometimes cancer of the lymph nodes. Death by the age of 40 is inevitable. XLP is an inherited defect due to a faulty gene on the X chromosome. Females have two X chromosomes, so if one copy is faulty, the other can usually compensate. But males have just one X chromosome and one Y. There is no backup X, hence the predominance of the disease in males. The gene responsible (SH2D1A or SAP) causes a failure in the communication between the cells of the immune system. A protein known as SLAM ("signalling lymphocyte activation molecule") stimulates activity and proliferation of cells in the immune system. SLAM is found on the surface of both B and T cells, effectively coupling them together. When this happens, it signals changes inside the cells that cause them to become active and to develop and proliferate. SAP ("SLAM-associated protein") is produced by T cells, and is an inhibitor of SLAM. This is the gene which is defective in XLP. HHV-4 first infects the throat but shortly afterwards invades the B cells, and triggers them to multiply, partly by increasing the number of SLAM proteins on the surface. Without functional SAP protein, the body is unable to control the B-cell proliferation triggered by infection. T cells recognize the EBV-infected B cells as foreign and instigate a massive inflammatory response (J.Sayos et al. Nature 395: 462-469, 1998).
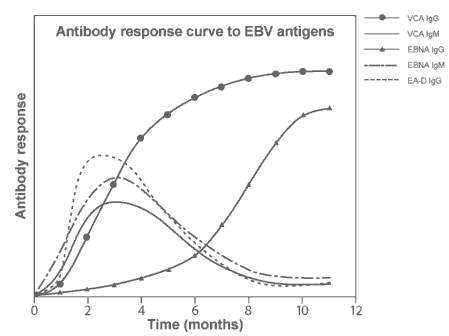 |
















